Developing an Innovative and De-Risked Pipeline
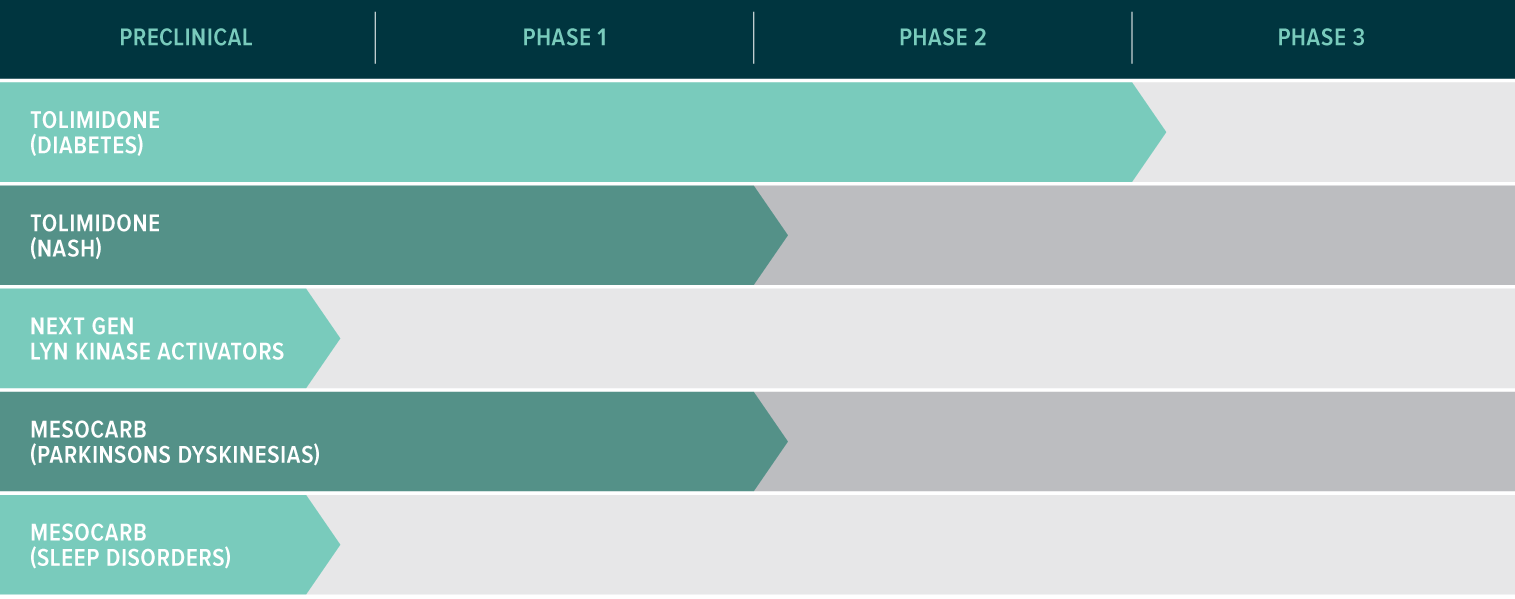
Tolimidone
A Safe and Well Tolerated Treatment for Metabolic and Liver Diseases
A new way to treat Type II Diabetes
- Positive clinical efficacy and remarkable safety and tolerability in a Phase 2a Type II diabetes study
- Over 300 clinical exposures to date
- 700 total clinical exposures expected by Q1 2019
- Additional safety data from a Phase 2b Type II Diabetes study expected Q1 2019.
Strong potential for clinical development in NASH
- Positive results in a comprehensive preclinical model of NASH
- Improvement in NAS score and key measures of NASH
Mesocarb
Addressing Unmet Needs in Parkinson’s Disease
- Mesocarb is a highly selective, well tolerated, dopamine reuptake inhibitor with strong preclinical data in Parkinson’s Dyskinesia
- A well characterized molecule that has been available commercially outside the United States
- Over 40 clinical publications
- Over one million patient-years exposure to date
